RBQM Risk Assessment Risk Management Central Monitoring Quality Tolerance Limits Site Management
Made Simple
Discover a smarter way to manage Risk-Based Quality Management (RBQM) with an easy-to-use platform that streamlines risk management and helps you take confident action when it matters most.

Focus. Confidence. Efficiency.


ABOUT OPRA
OPRA, Pioneering RBQM for Clinical Trial Excellence
OPRA is an innovative platform designed to simplify Risk-Based Quality Management (RBQM) for clinical trials of any scale. With its core modules, OPRA-RAM and OPRA-CM, teams can seamlessly manage all risks, controls, and actions in one place. Whether used independently or together, OPRA enables teams to focus on what matters most, prioritize critical risks, and take confident, timely action - all backed by clear, evidence-driven insights.
-
Deployed on over 450 clinical trials
-
Secure, Validated, 21 CFR part 11 compliant
-
Designed for Sponsors and CROs running all trial phases

CENTRALIZED MONITORING
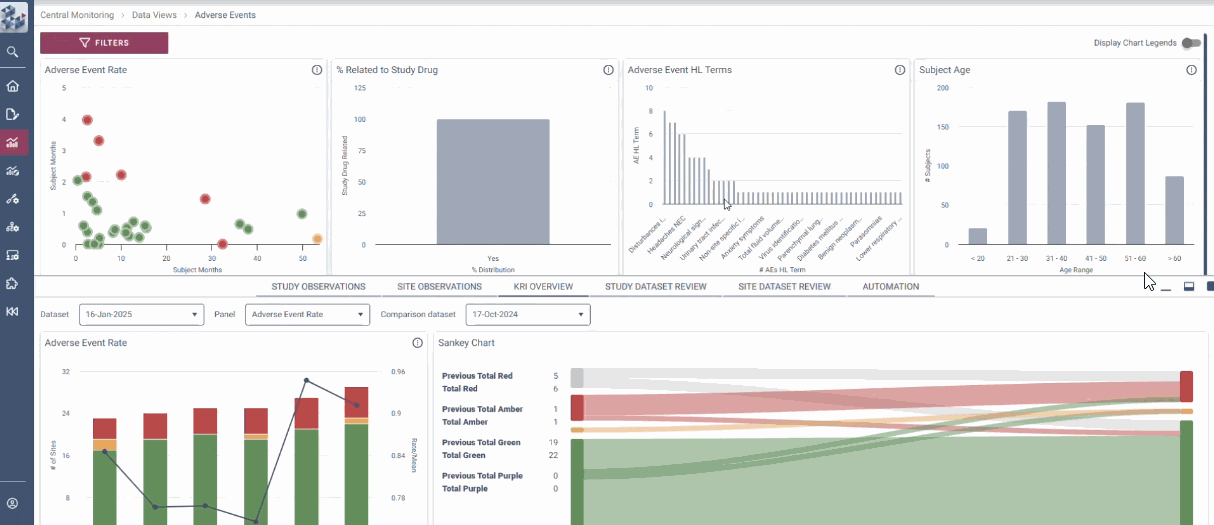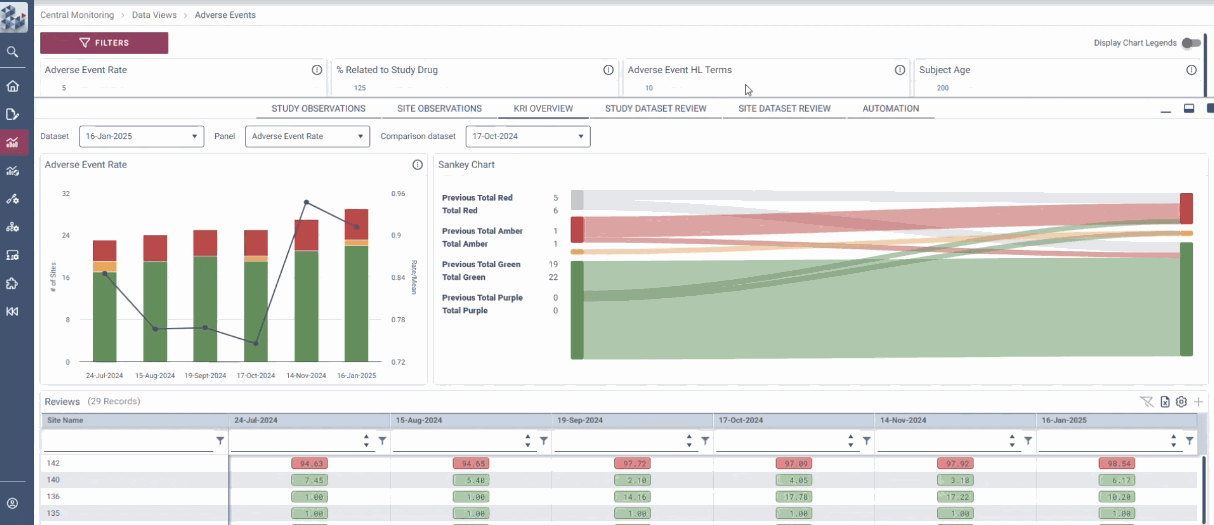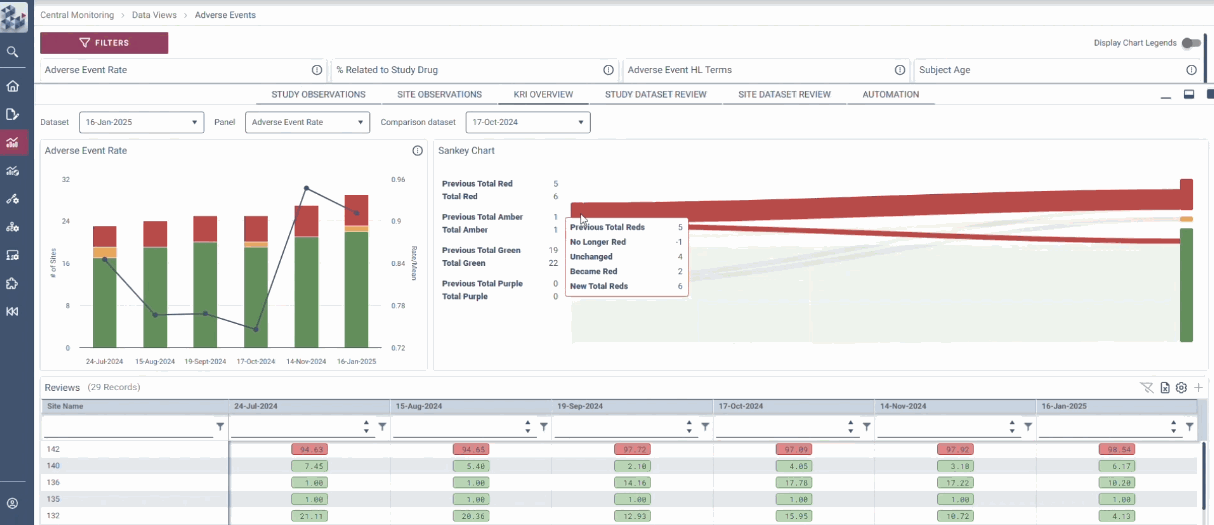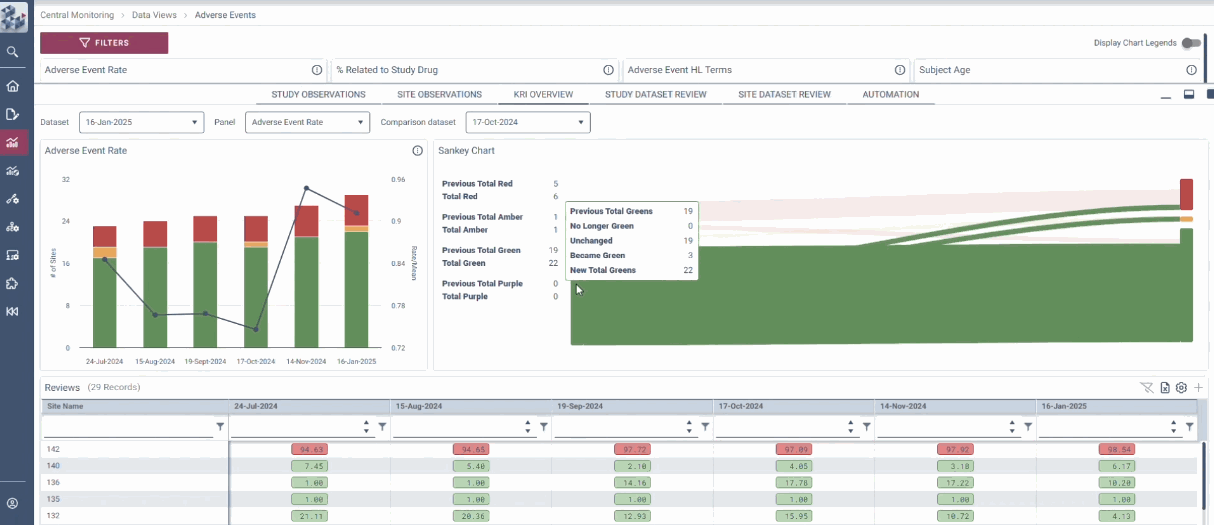
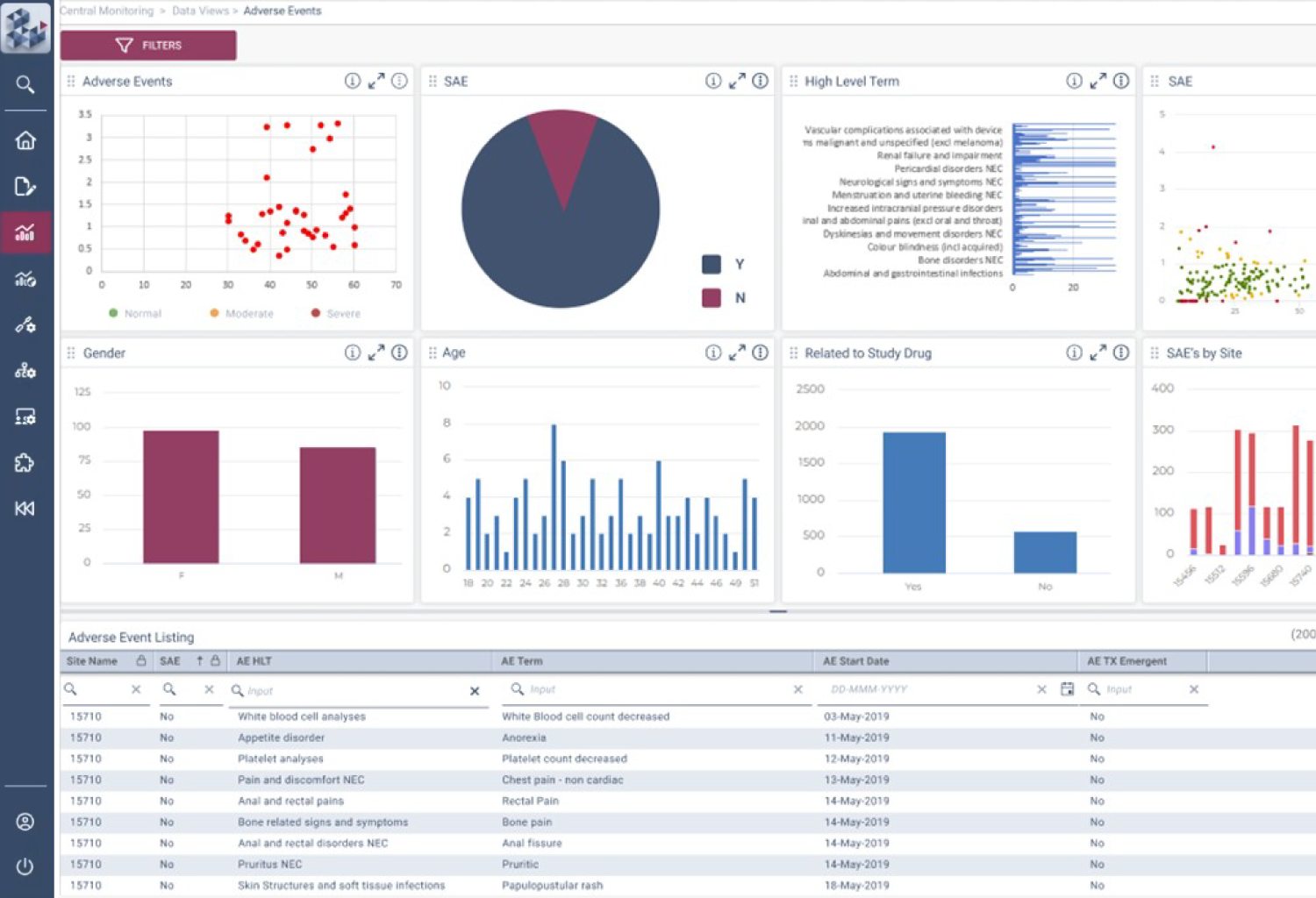
Centralized Monitoring, Reimagined
OPRA-CM redefines clinical trial monitoring with an efficient, evidence-driven approach to RBQM. Designed by Clinical Operations experts, it eliminates the burdens of conventional monitoring, enabling teams to visualize trends, track changes, and proactively identify and mitigate risks - all in one place. Tailored to modern trials of any scale, phase, or location, OPRA-CM keeps teams focused on what matters most, ensuring confidence and control at every step.
-
Enable instant focus into what matters most
-
Make data-driven decisions with confidence
-
Achieve efficiency gains of up to 30%.
RISK ASSESSMENT AND MANAGEMENT
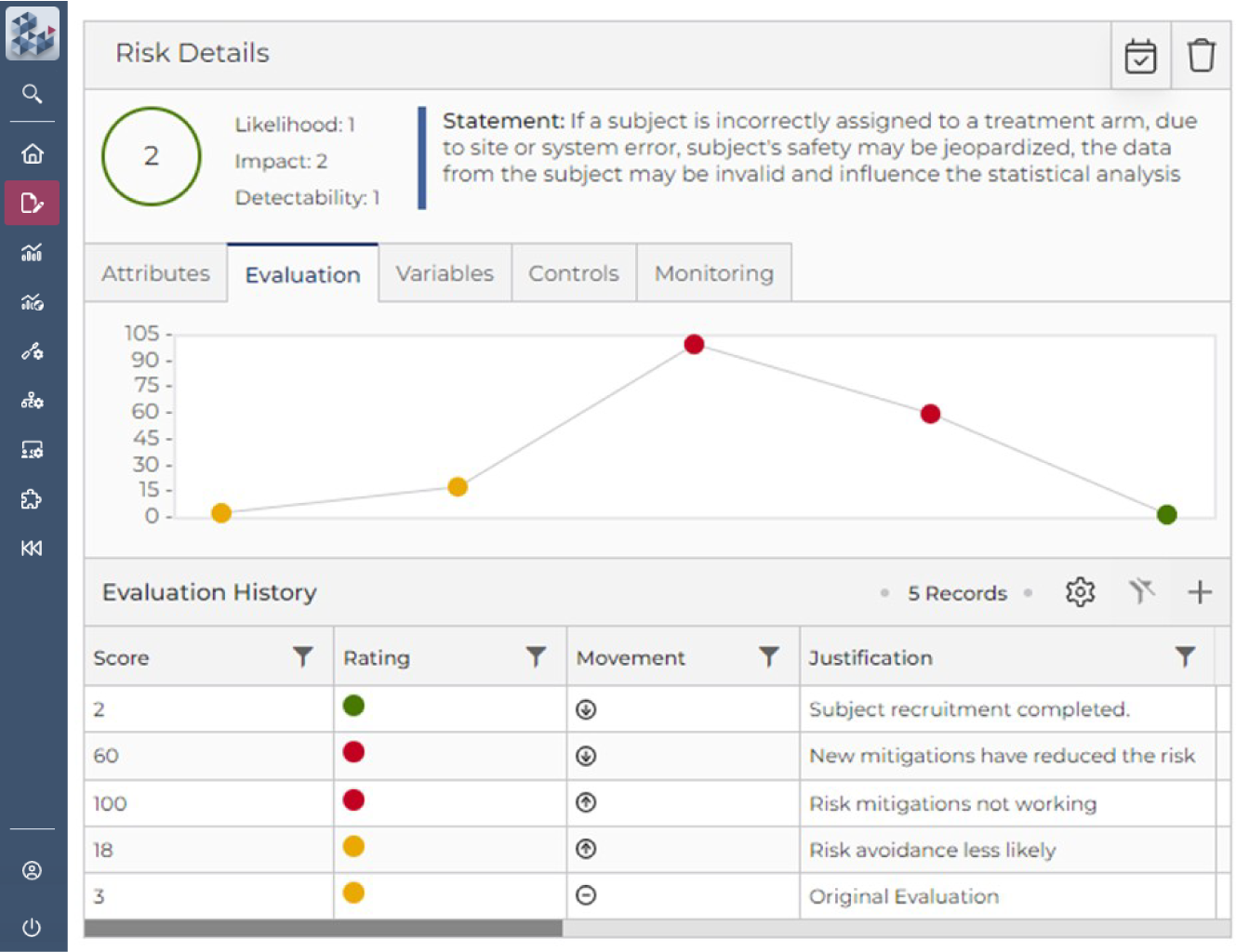
Risk Assessment & Management Simplified
OPRA-RAM is a single, user-friendly platform to manage all risks, controls, and actions and quickly identify key areas of focus. By centralizing activity management, teams can establish clear risk-variable relationships, monitor trends over time, and assess the effectiveness of controls—more efficiently than ever before. Suitable for trials of any scale, phase, or location, OPRA-RAM streamlines risk assessment and management to keep studies on track and ensure compliance.
-
Quickly prioritize risk and implement controls
-
Focus resource on critical risks and activities
-
Demonstrate proactive risk management
SUBJECT MONITORING
Subject Monitoring Precision in RBQM
In early phase trials, the ability to oversee the safety and progress of each patient is paramount. OPRA-SM is designed to idenitify and manage patient risk, where traditional site-based Central Monitoring approaches don't work. Medical monitoring of patient data can be inefficient and error-prone, but those problems go away with OPRA-SM's unique views on data changes and patient journeys.
-
Enhanced patient safety
-
Improved data integrity
-
Greater operational efficiency
Compare OPRA
to traditional monitoring solutions
Traditional Monitoring
OPRA
Proactive
Proactively identifies and addresses risks throughout the trial, preventing issues before they occur.
Comprehensive
Takes a holistic approach, considering risks across the entire trial, from protocol design to data analysis.
Data-Driven
Relies on data and analytics to make informed decisions about risk mitigation.
Flexible
Adapts to changes and unforeseen circumstances, maintaining quality in trials.
Adaptable
Allows for tailored risk assessments and mitigation strategies based on trial-specific factors.
Transparent
Promotes transparency by documenting risk assessments, mitigation plans, and decision-making processes.
Frequently asked questions
We've got the answer
Explore our FAQs for answers and expert advice on RBQM.
TESTIMONIALS
RBQM Success Stories
Hear directly from our partners about how we've helped them.


What truly set TriTrials apart from other vendors was their unparalleled commitment to a quality deliverable helping us effectively apply a novel approach to our operations. They achieved a deep understanding of our objectives by partnering with us on a rigorous change management process which culminated in a shared vision for the integration of their risk assessment and central monitoring solution.

The Worldwide Clinical Trials team is pleased with our continued partnership with TRI and we are excited about the milestone of having 150 clinical trials in TRI's Risk-Based Quality Management platform. This is a key benefit for our customers as it improves data quality, trial efficiency, and patient safety.
RBQM NEWS
Clinical trial quality news and views
Subscribe to get the latest RBQM insights sent to your mailbox.
Join Us at the SCDM 2025 EMEA Conference We’re excited to announce that TRI – The RBQM Experts will be exhibiting at the SCDM 2025 EMEA Conference in Brussels, Belgium,…
Reflecting on Two Great Conferences – Let’s Talk! In this edition of the Quality Issue, we’re taking a moment to look back on two fantastic conferences we’ve recently attended. Too…
Happy New Year, and welcome to our first Quality Issue of 2025! It this edition, we spotlight some of the most important developments in clinical research and Risk-Based Quality Management…










