Risk Management
Made Simple
Adopt a smarter approach to clinical trial risk management with OPRA-RAM, where every decision is informed, every risk is managed, and every trial sets a new standard in excellence.





ABOUT OPRA RAM
Makes clinical trial risk management workflows, flow
Introducing OPRA-RAM by TRI – your gateway to next-level clinical trial risk management. OPRA-RAM is about more than managing risks; it's about integrating quality into every aspect of your trial. Inspired by the principles of ICH E6 and ICH E8, OPRA-RAM brings a systematic quality focus that aligns with GCP standards.
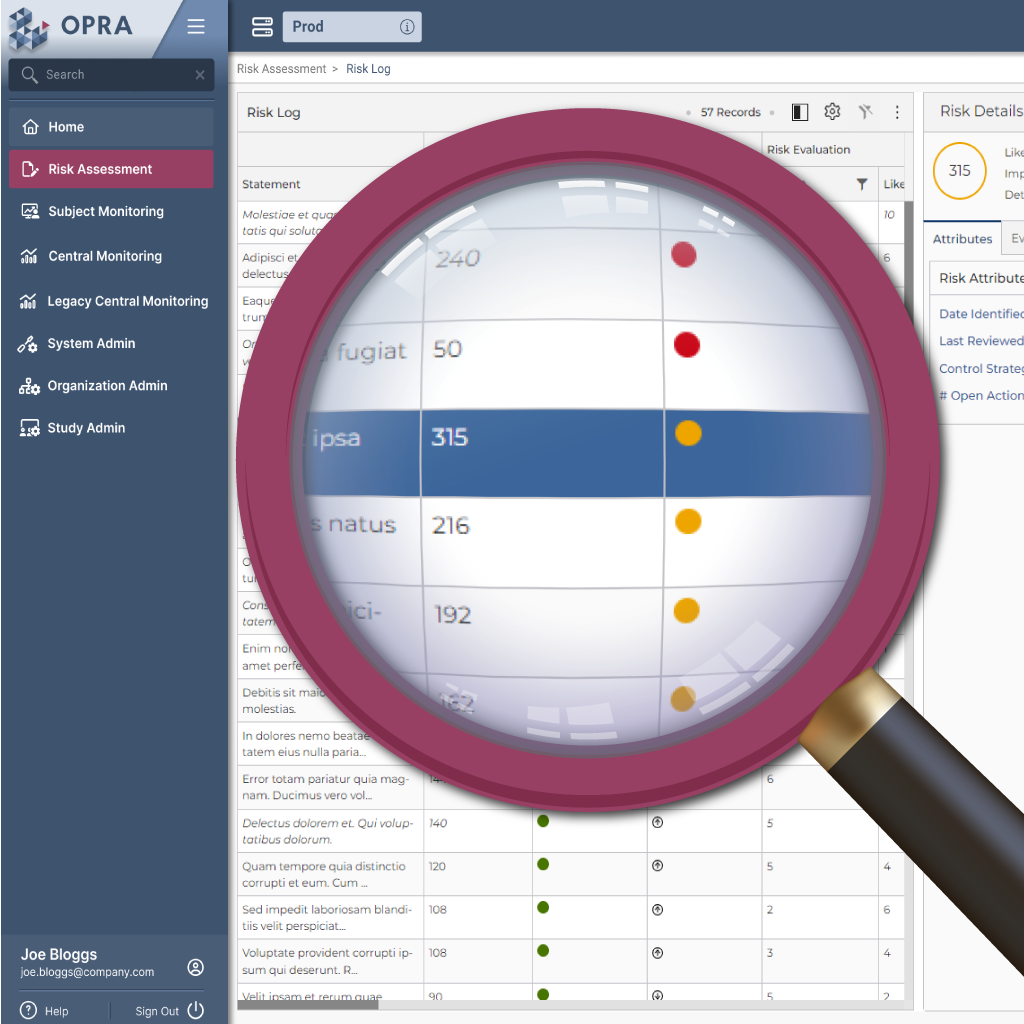
The foundation of clinical trial quality is understanding and communicating the relationship between critical data, risks to that data, and mitigations (or controls) to those risks. OPRA-RAM is a centralized, cloud-driven platform meticulously designed to navigate the complexities of critical data, risks, and controls with ease. It empowers stakeholders to access, analyze, and mobilize essential insights from any location, ensuring decisions are not just timely but transparent and centralized. By bringing these vital elements under one platform, OPRA-RAM refines processes, boosts transparency, and nurtures a collaborative space, driving enhanced efficiency and effectiveness in clinical trial risk management.
OPRA-RAM's Risk Scoring feature is all about customization and precision, tailored for the unique contours of clinical trials. Inspired by the TransCelerate RACT model, which sizes up risks by impact, likelihood, and detectability, OPRA-RAM puts you in the driver's seat. You get to pick how detailed your scoring gets - from a straightforward 1-3 scale to a more nuanced 1-10. This flexibility means your risk scoring can match your trial's specific needs. Plus, with OPRA-RAM, setting your own risk thresholds is a breeze. You'll have a clear, color-coded map of what's critical (red), what needs a heads-up (amber), and what's smooth sailing (green) right on the platform, making those big decisions a little bit easier.
Featuring a dynamic Risk and Controls Library, OPRA-RAM is designed to capture enterprise knowledge and experience, to provide users with insights on risks that are critical to quality, based on key study parameters such as study phase and therapy area. Users can customize their clinical trial risk management approach to fit each unique study. Each risk in the library can be associated with one or more suggested risk controls, giving further insight into what controls have worked well on previous studies. With the ability to import from risk libraries or build from scratch, OPRA-RAM offers unparalleled flexibility.
Excel risk assessment is notorious for version control challenges, lack of audit trial, lack of validation and inability to centralize and collaborate. OPRA-RAM solves all these issues, pioneering a new era in clinical trial risk management. Through its role-based permissions, OPRA-RAM ensures that every user gain access only to the parts pertinent to their role in risk management, promoting transparency, collaboration and a single source of truth across the enterprise, and between Sponsors, CROs and vendors.

ABOUT OPRA RAM
Proactive clinical trial risk management
OPRA-RAM presents a suite of features crafted to do more than just respond to risks – it's engineered to anticipate and effectively mitigate them. It's all about elevating your trials with heightened foresight and proactive measures, ensuring that decisions are well-informed and pre-emptive of potential hurdles. With OPRA-RAM, you're embracing a refined and intuitive approach to navigating clinical trial risk management.
Harness the power of real-time insights with OPRA-RAM's ready-made Risk Dashboards. These dashboards are expertly designed to provide an instant overview of your clinical trial's risk landscape, presenting key insights at a glance. Effortlessly monitor key risk trends, track the progress of mitigation strategies, and make informed decisions swiftly.
OPRA-RAM enhances clinical trial risk management through its robust activity management feature, empowering users to monitor the progression of risk scores, reviews and risk-management activities over time. This invaluable tool not only highlights changes since the last risk assessment but also offers a comprehensive view of impact, likelihood, and detectability scores as they evolve. By visualizing risk trajectories, OPRA-RAM facilitates an enhanced understanding of the efficacy of mitigation strategies and allows for more predictive approach to risk management and mitigation to be taken.
Featuring in-built review cycles, OPRA-RAM enables continuous evaluation of the evolving risk landscape of your clinical trials. With the flexibility to make comments, record observations, and adapt risk scores as necessary, you maintain a living record of your risk management efforts. This ongoing assessment ensures that your approach to risk is as adaptive and responsive as the trials themselves, allowing for real-time adjustments and informed decision-making.
ABOUT OPRA RAM
Clinical trial risk management reporting, your way
OPRA RAM is where clarity meets compliance in clinical trial risk management. With advanced reporting capabilities, you're equipped to communicate risk more effectively and efficiently, stripping away the complications of traditional reporting methods. Tailor your reports to the needs of your stakeholders with ease, ensuring that every piece of information is clear, concise, and impactful.
Unlock seamless risk communication with OPRA RAM's advanced reporting capabilities. This feature streamlines the way you share and communicate risk information, eradicating the complexities associated with traditional document sharing. With options for generating PDFs and bespoke reports, OPRA RAM ensures that you can tailor your risk communication to meet the specific needs of your stakeholders. These advanced reporting tools not only enhance clarity and understanding but also facilitate a more efficient exchange of critical risk-related data.
For Enterprise customers, you can benefit from permanent read-only access to your study data even after its conclusion, ensuring you're always prepared for regulatory scrutiny and questions. This enduring availability not only supports seamless compliance but also creates a comprehensive record of what transpired and the rationale behind any changes, facilitating a smooth review process when submitting clinical trial results.
OPRA-RAM's one-click reporting simplifies the way you access and compile data, turning hours of work into a mere moment's task. With just one click, you can generate comprehensive reports that combine accuracy with clarity, ensuring you spend less time on data processing and more time on decision-making. Reports can be stored within the platform, giving a full history of reporting over the lifetime of the trial, or exported for inclusion in the TMF.

Compare
Clinical Trial Risk Management Strategies
Spreadsheets
Data Visualization Tools
OPRA-RAM
Real-time insights
Availability of up-to-date risk scores
Document corrective actions
Document key information
Collaboration
Centralized portal to collaborate with stakeholders
Trend analysis
View evolution of risks over time
Alerts
Customizable alerts
Data visualizations
Customizable data visualizations
Version control
Eradicates duplication
Document corrective actions
Document key information
Assign actions
Assign actions to stakeholders
Audit ready
Full discoverability of mitigations after closeout
FAQs
We've got the answer
Explore our FAQs for answers and expert advice on RBQM.
TESTIMONIALS
RBQM Success Stories
Hear directly from our partners about how we've helped them.


What truly set TriTrials apart from other vendors was their unparalleled commitment to a quality deliverable helping us effectively apply a novel approach to our operations. They achieved a deep understanding of our objectives by partnering with us on a rigorous change management process which culminated in a shared vision for the integration of their risk assessment and central monitoring solution.

The Worldwide Clinical Trials team is pleased with our continued partnership with TRI and we are excited about the milestone of having 150 clinical trials in TRI's Risk-Based Quality Management platform. This is a key benefit for our customers as it improves data quality, trial efficiency, and patient safety.
RBQM NEWS
Clinical trial quality news and views
Subscribe to get the latest RBQM insights sent to your mailbox.
The Quality Issue – January 2025
Happy New Year, and welcome to our first Quality Issue of 2025! It this edition, we spotlight some of the most important developments in clinical research and Risk-Based Quality Management…
Dose Optimization in Oncology: Advancing Clinical Trial Quality through Risk-Based Approaches
The complexities of oncology drug development have long posed significant challenges, particularly in determining the optimal dose that balances therapeutic efficacy with patient safety. The FDA’s recent guidance on dose…
TRI Joins Veeva’s Product Partner Program
Partnership will drive quality and efficiency in clinical trials through enhanced risk-based quality management integration. [Cambridge, UK – 17.10.2024] – TRI, a leading Risk-Based Quality Management (RBQM) software provider for…









