Risk Management
Made Simple
Discover a simpler way to manage clinical trial risk. OPRA-RAM is a user-friendly platform that centralizes risk assessment and management so teams can quickly identify, track, and mitigate study risks.

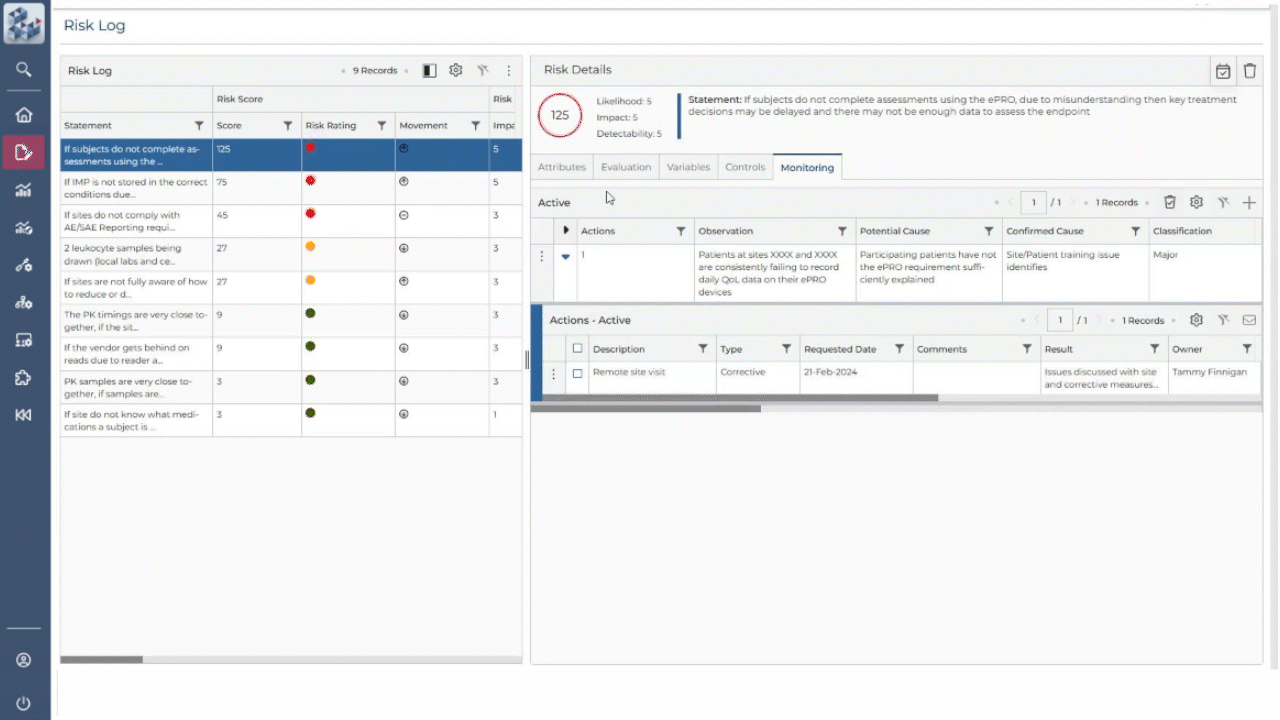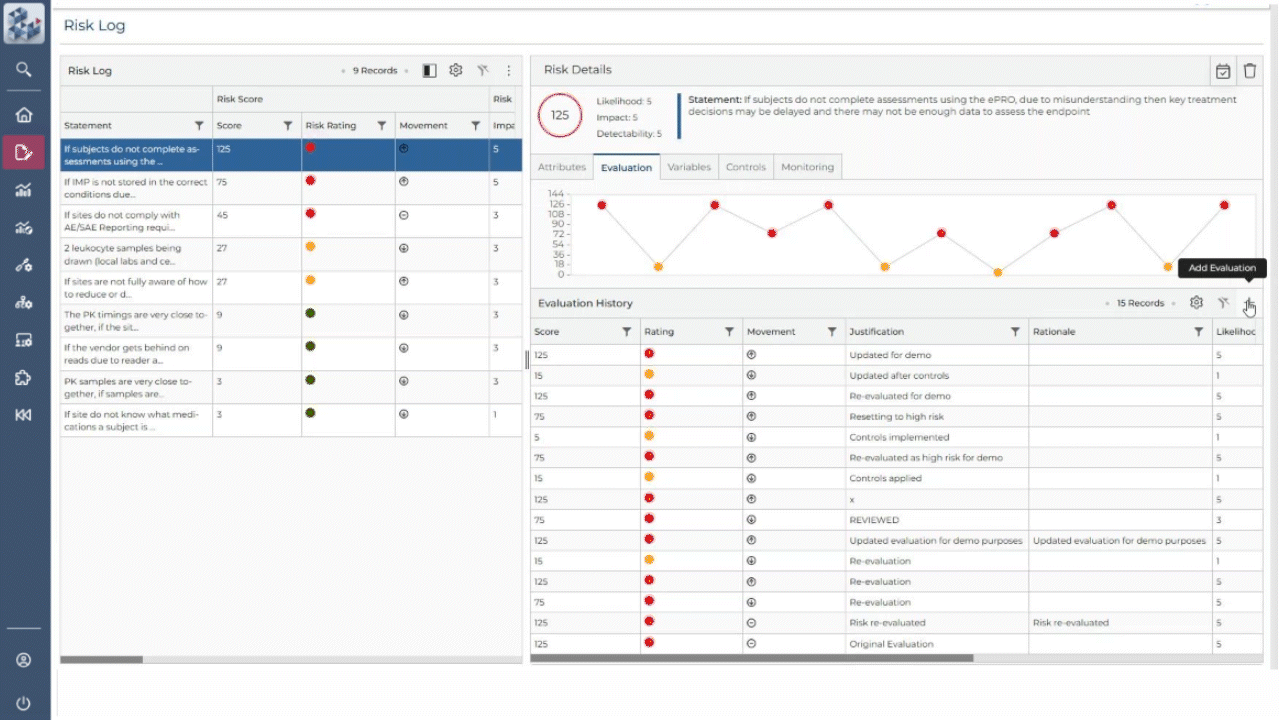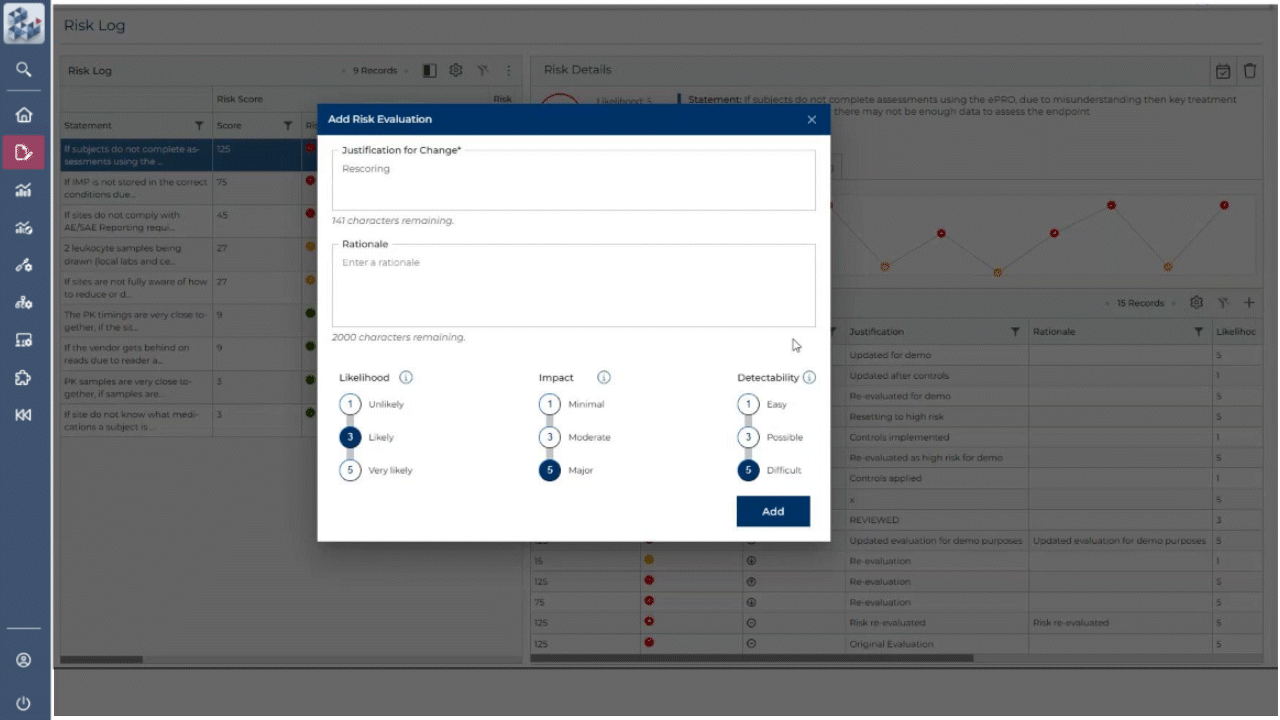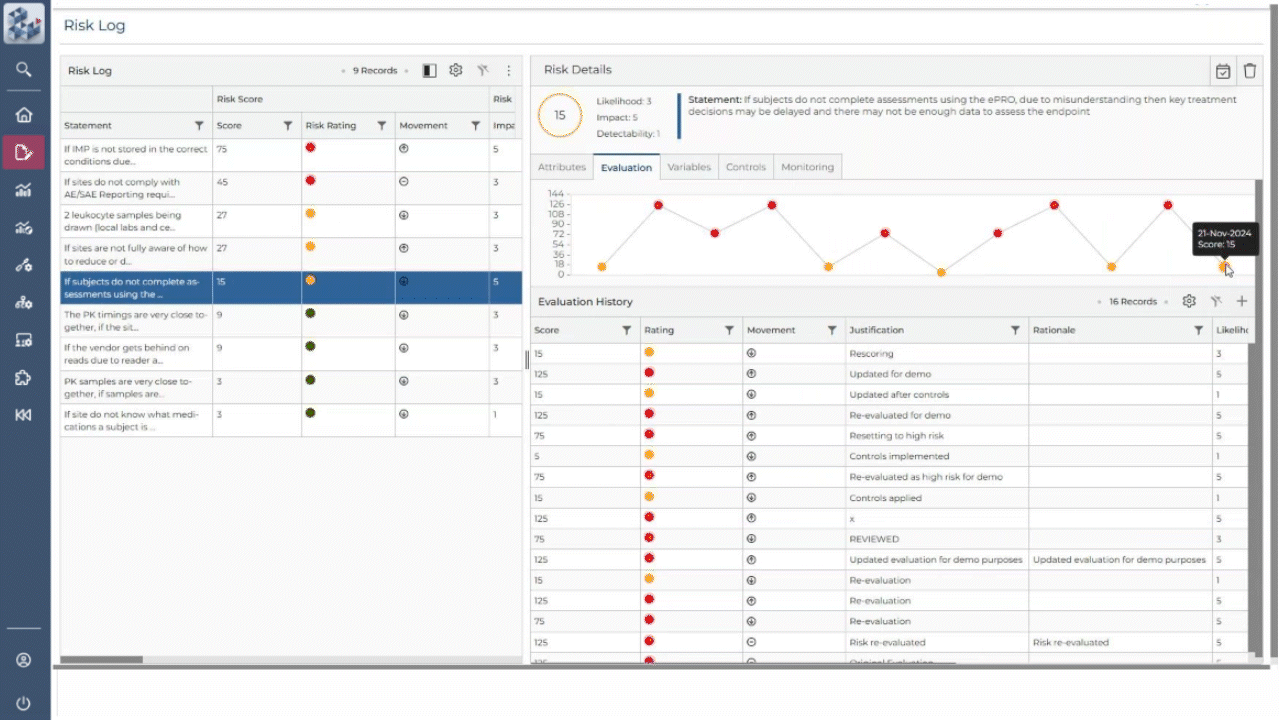

ABOUT OPRA RAM
Risk management, simplified
OPRA-RAM transforms risk assessment and management by consolidating all activities into a single platform. Designed for ease of use, it allows teams to quickly prioritize risks, implement controls, and maintain oversight with confidence. By centralizing processes and promoting proactive risk management, OPRA-RAM ensures that studies stay on track and compliant.
-
Focus
-
Confidence
-
Efficiency
Focus
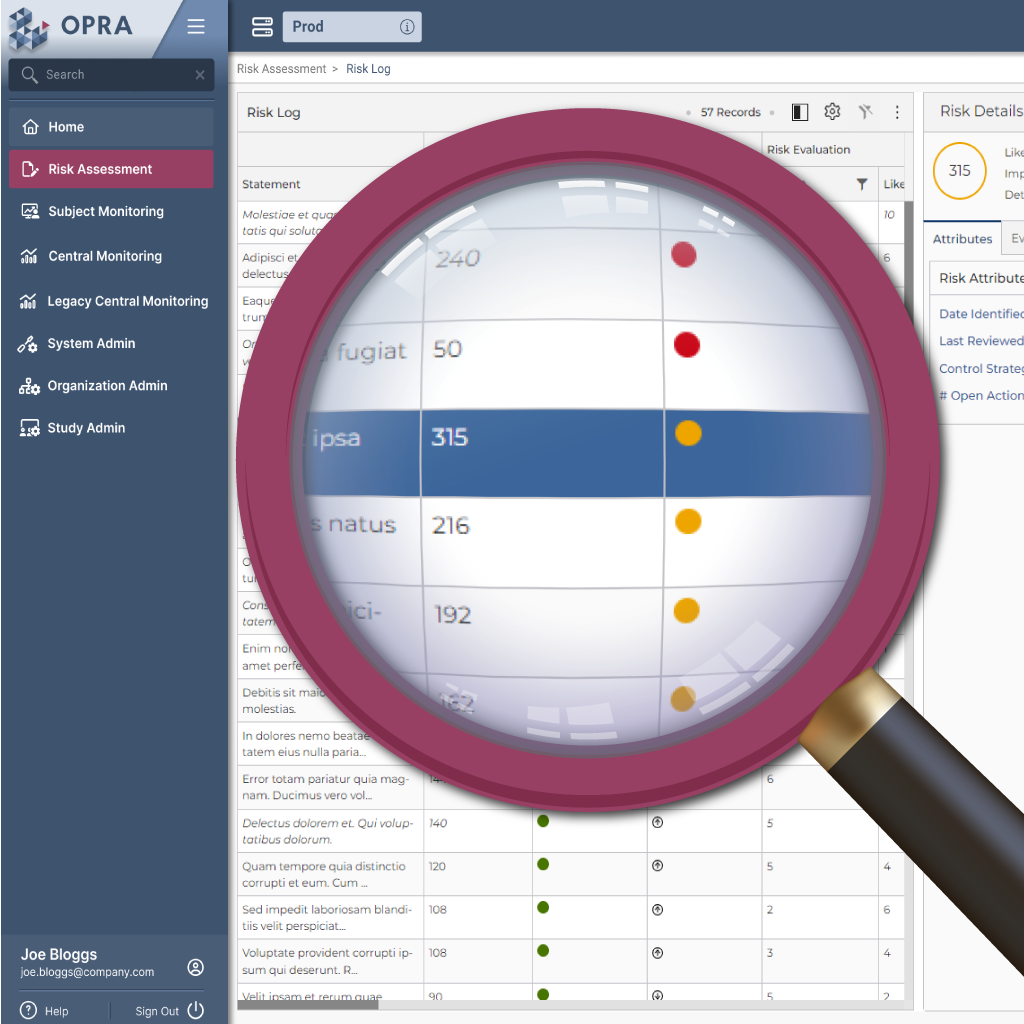
Stay ahead of study risks
Cut through complexity to stay focused on what matters. OPRA-RAM centralizes risk assessment and management, replacing outdated, manual processes with a streamlined, intuitive system. By bringing all risks, controls, and actions into one place, teams quickly prioritize critical risks, assign ownership, and track trends to maintain oversight and take proactive action.
-
Manage all risks, controls, and actions in one place
-
Easily score and prioritize critical risks
-
Quickly identify key areas of focus
Confidence
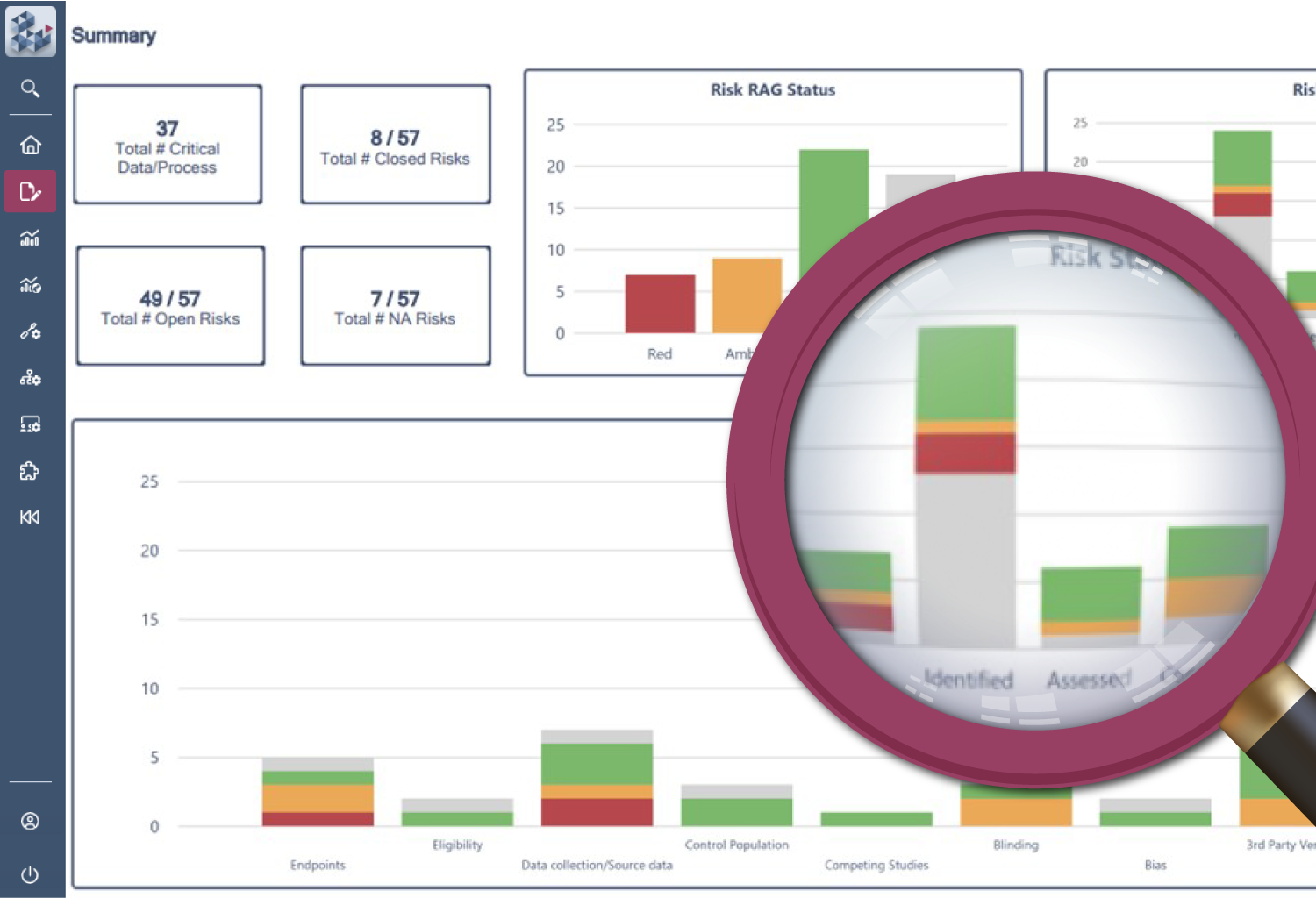
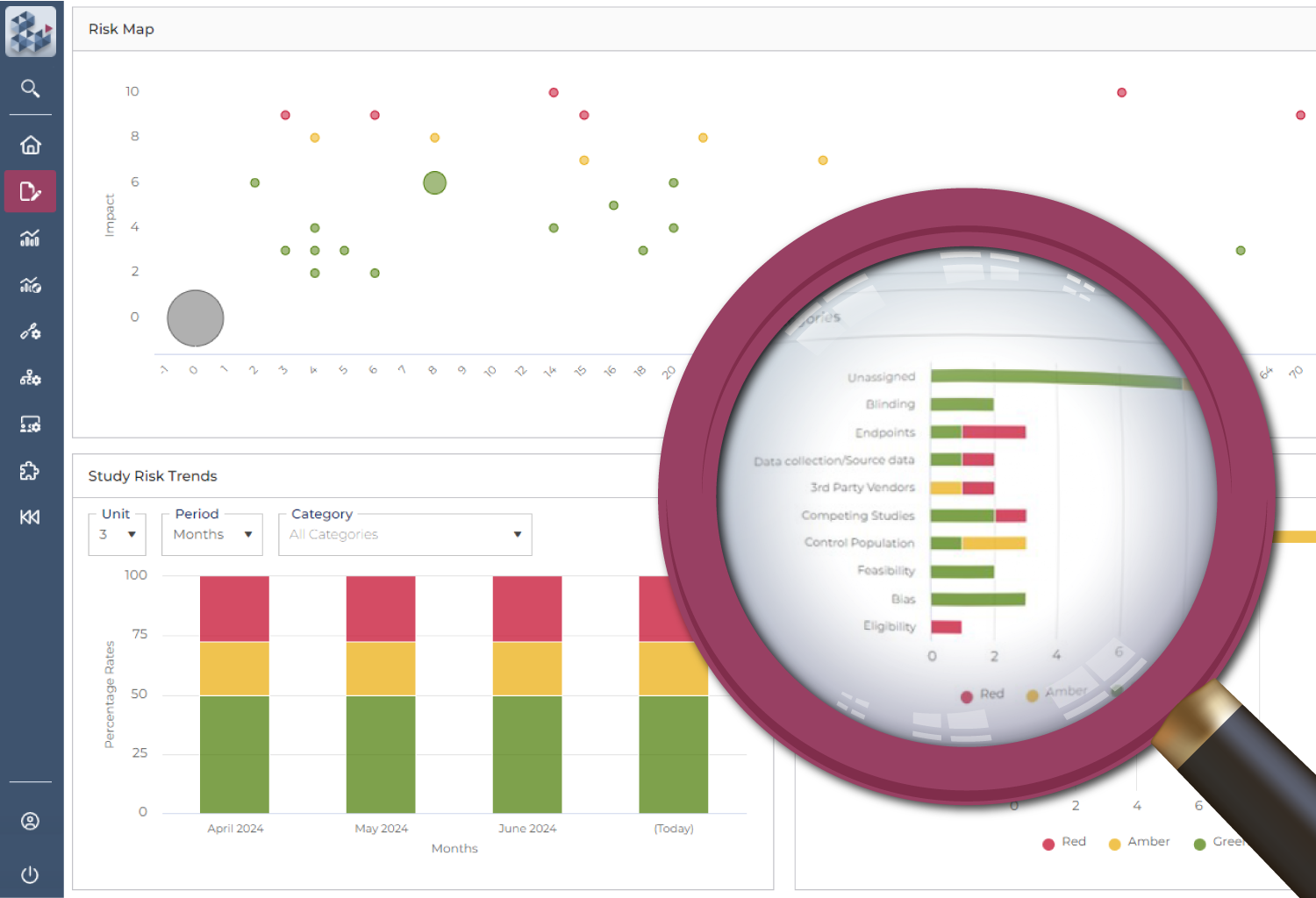
Ensure compliance and control
Outdated systems make it difficult to demonstrate that risk management is being carried out effectively. OPRA-RAM ensures compliance with a validated, centralized system that applies standardized risk libraries and best practices. By tracking trends and maintaining clear risk-variable relationships, teams can be confident that risks are well-managed and documented.
-
Establish clear risk-variable relationships
-
Track trends over time
-
Demonstrate proactive risk management
Efficiency
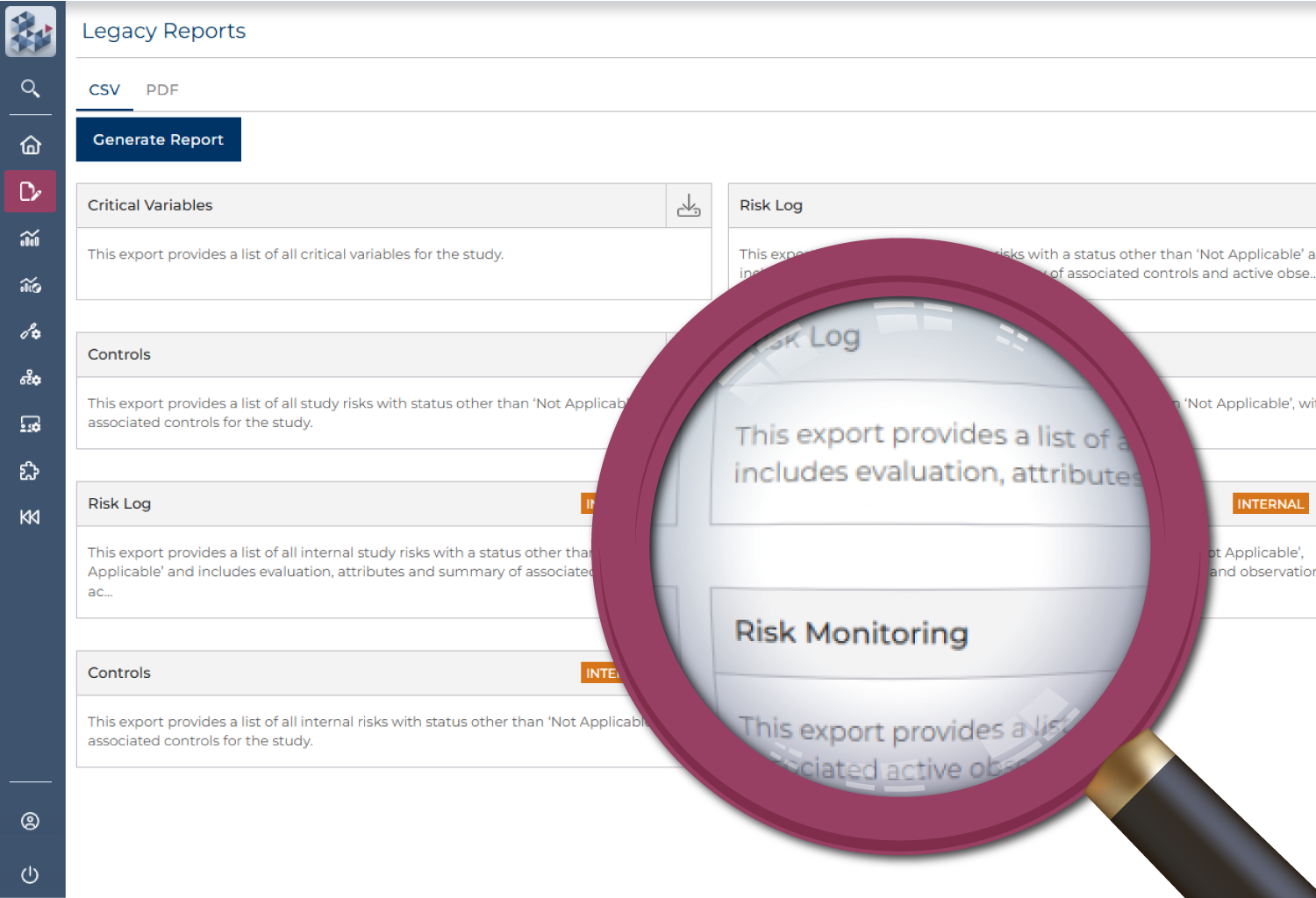
Efficiency without complexity
With limited resources and growing oversight responsibilities, teams need a smarter way to manage risk. OPRA-RAM streamlines risk assessment and management, accelerating setup and keeping studies on track through a central, easy-to-use portal. Centralized actions, reusable risk libraries, and automated alerts reduce manual effort—so teams can focus attention where it is needed most.
-
Create risk libraries to streamline setup
-
Centralize activity management
-
Quickly prioritize risk and implement controls
Compare
Clinical Trial Risk Management Strategies
Spreadsheets
Data Visualization Tools
OPRA-RAM
Real-time insights
Availability of up-to-date risk scores
Document corrective actions
Document key information
Collaboration
Centralized portal to collaborate with stakeholders
Trend analysis
View evolution of risks over time
Alerts
Customizable alerts
Data visualizations
Customizable data visualizations
Version control
Eradicates duplication
Document corrective actions
Document key information
Assign actions
Assign actions to stakeholders
Audit ready
Full discoverability of mitigations after closeout
FAQs
We've got the answer
Explore our FAQs for answers and expert advice on RBQM.
TESTIMONIALS
RBQM Success Stories
Hear directly from our partners about how we've helped them.


What truly set TriTrials apart from other vendors was their unparalleled commitment to a quality deliverable helping us effectively apply a novel approach to our operations. They achieved a deep understanding of our objectives by partnering with us on a rigorous change management process which culminated in a shared vision for the integration of their risk assessment and central monitoring solution.

The Worldwide Clinical Trials team is pleased with our continued partnership with TRI and we are excited about the milestone of having 150 clinical trials in TRI's Risk-Based Quality Management platform. This is a key benefit for our customers as it improves data quality, trial efficiency, and patient safety.
RBQM NEWS
Clinical trial quality news and views
Subscribe to get the latest RBQM insights sent to your mailbox.
The Quality Issue – January 2025
Happy New Year, and welcome to our first Quality Issue of 2025! It this edition, we spotlight some of the most important developments in clinical research and Risk-Based Quality Management…
Dose Optimization in Oncology: Advancing Clinical Trial Quality through Risk-Based Approaches
The complexities of oncology drug development have long posed significant challenges, particularly in determining the optimal dose that balances therapeutic efficacy with patient safety. The FDA’s recent guidance on dose…
TRI Joins Veeva’s Product Partner Program
Partnership will drive quality and efficiency in clinical trials through enhanced risk-based quality management integration. [Cambridge, UK – 17.10.2024] – TRI, a leading Risk-Based Quality Management (RBQM) software provider for…









