Centralized Monitoring
Made Simple
Elevate your trial oversight. OPRA-CM enhances centralized monitoring for clinical trials with a smarter and more efficient RBQM approach, simplifying complexity to proactively identify risks and take faster action.

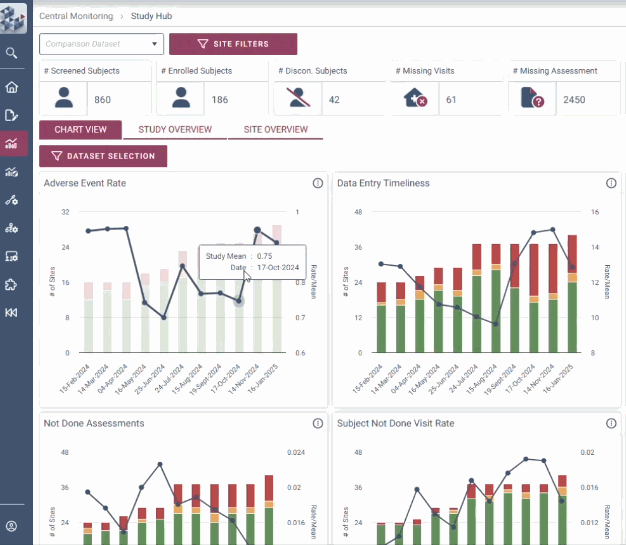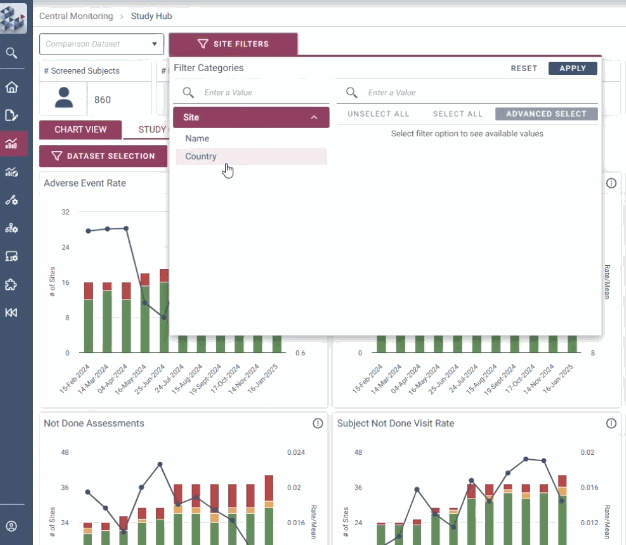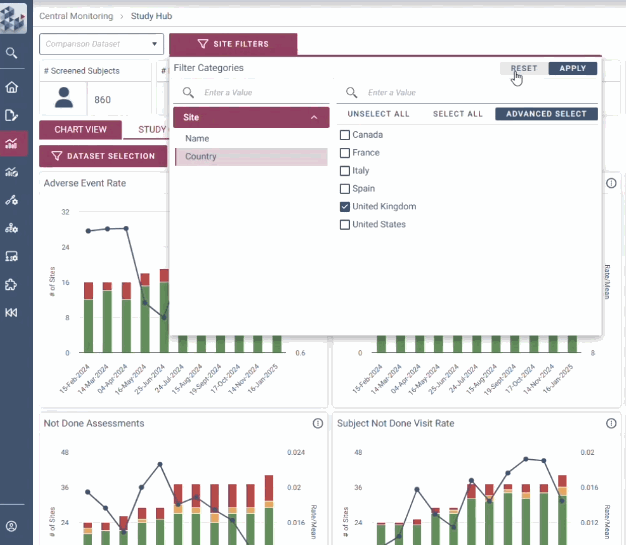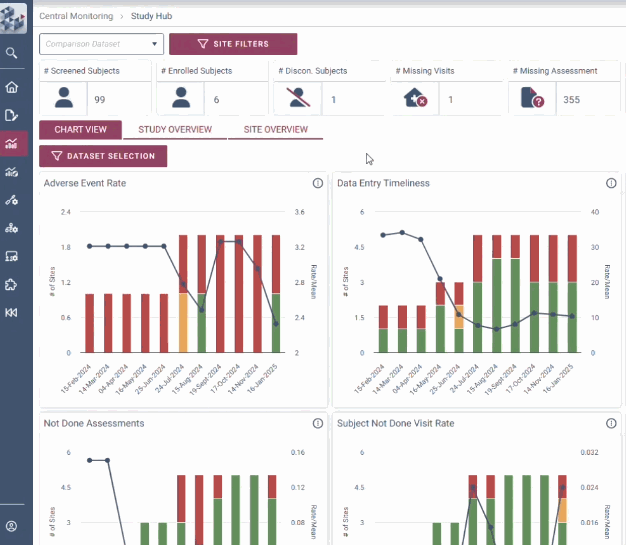

ABOUT OPRA-CM
Centralized monitoring, reimagined
OPRA-CM transforms centralized monitoring by providing instant focus on critical risks, instilling confidence in decision-making, and driving efficiency improvements of up to 30%.
The easy-to-use platform empowers teams to streamline risk-based monitoring and take timely, data-backed actions with clarity and confidence.
-
Focus
-
Confidence
-
Efficiency
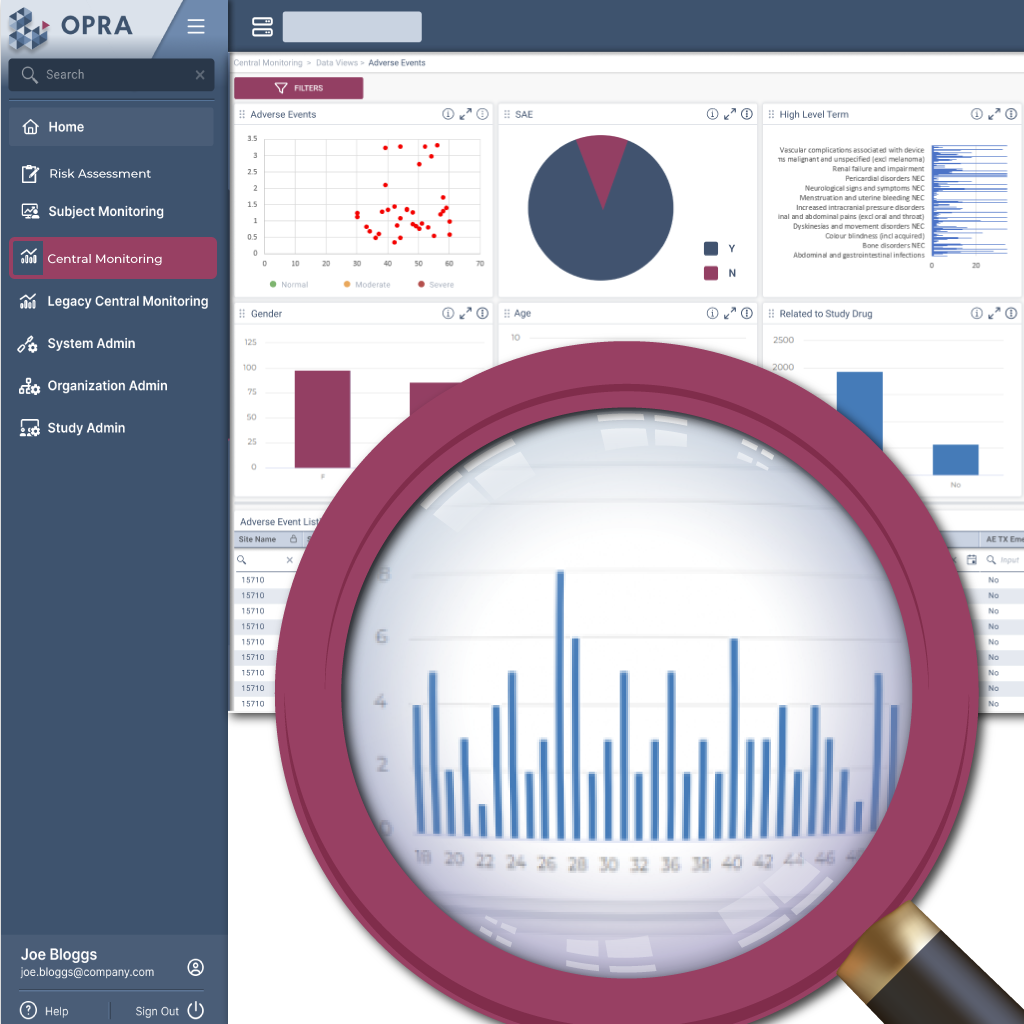
Focus
Focus on critical risks
OPRA-CM goes beyond simply aggregating data - it directs attention to what truly matters. Instead of overwhelming users with endless charts and metrics, it highlights key areas of concern, guiding teams to the insights that require action. With a clear view of trends over time and the ability to drill down into specific risks, teams can quickly monitor clinical trial risk and take appropriate action.
-
Instantly visualise changes over time
-
Uncover hidden insights
-
Quickly identify areas of concern
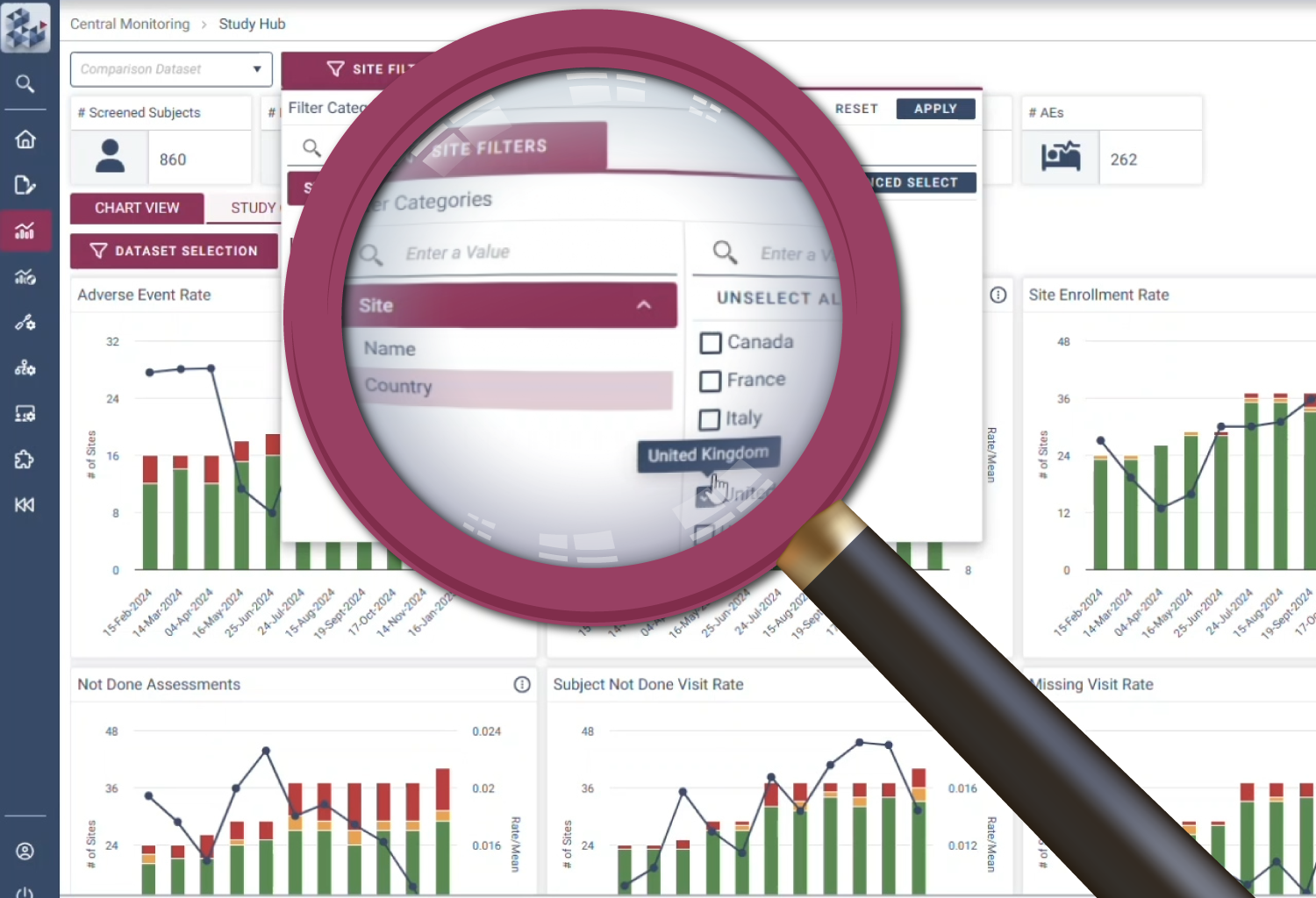
Confidence
Make assured decisions
Decision-making is at the heart of RBQM. OPRA-CM removes uncertainty with a dynamic view of risk indicators that track trends over time instead of relying on static snapshots. Its intelligent data structure overlays key metrics to provide context and help teams adapt to emerging risks. With complete visibility into study, site, and subject data, teams can make informed decisions with clarity and certainty.
-
Ensure complete data visibility
-
Adapt to emerging risks and changes
-
Facilitate evidence-based decision making
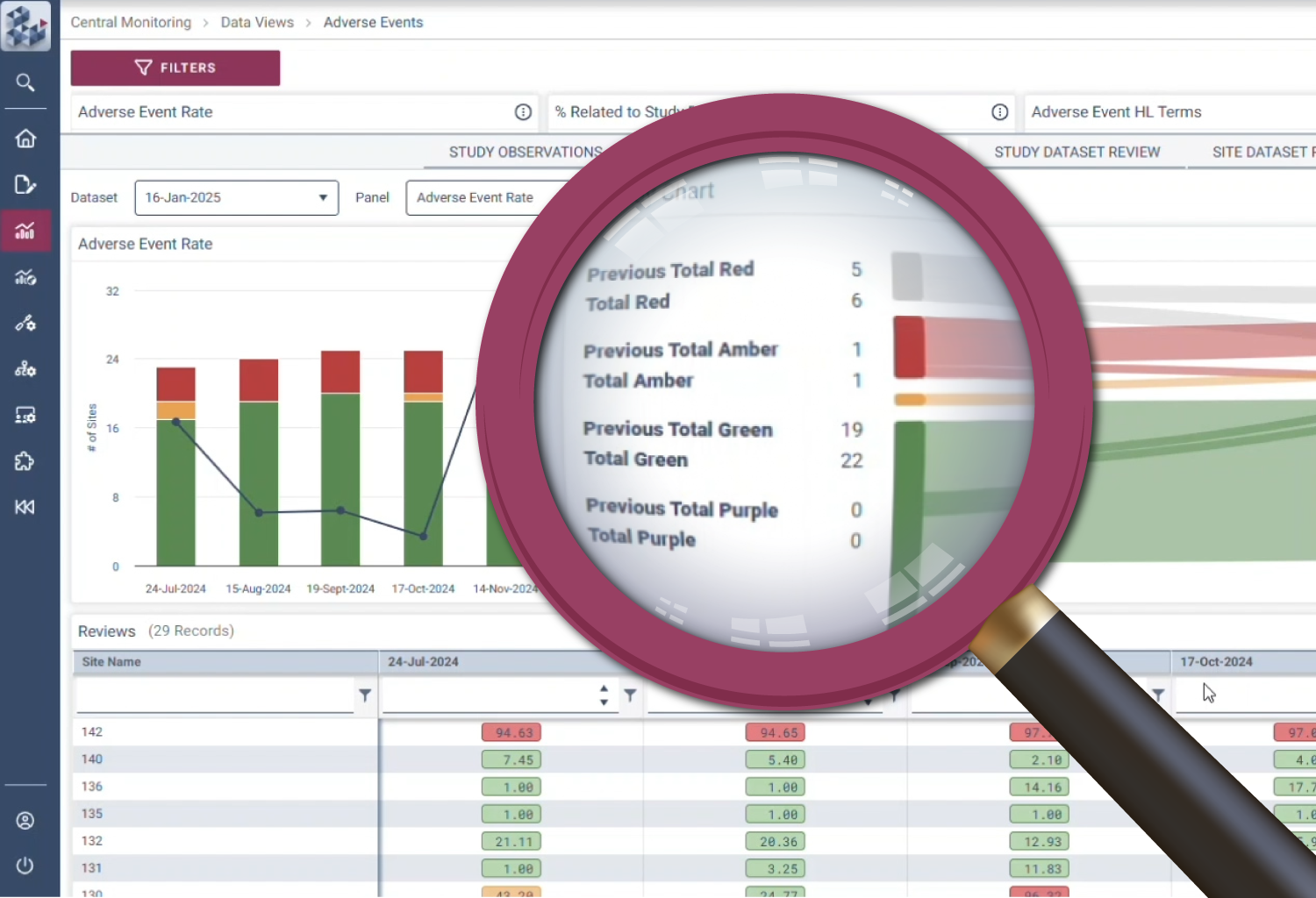
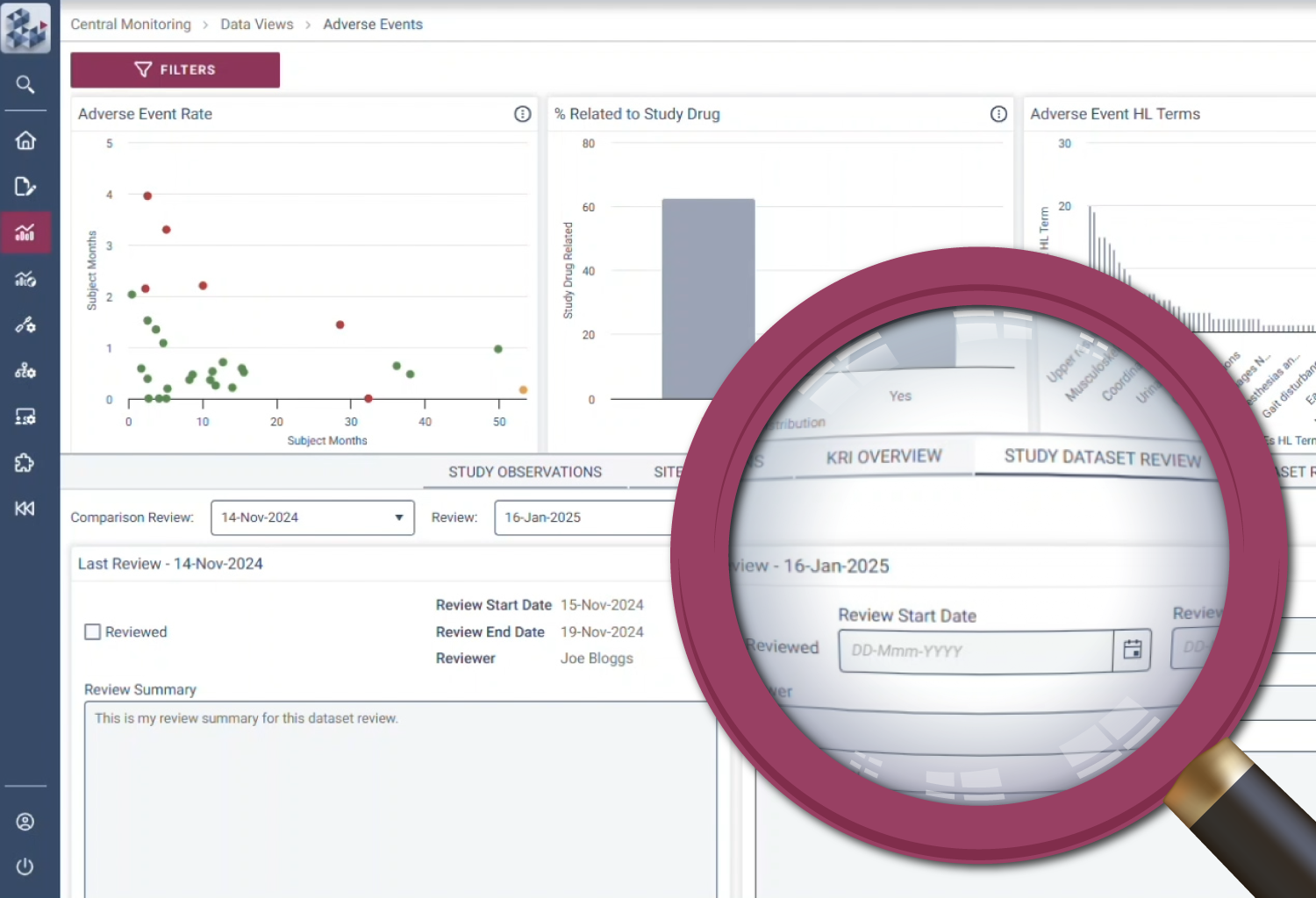
Efficiency
Be more effective
With growing pressure to do more with less, OPRA-CM lets technology do the heavy lifting. Pre-defined workflows automatically create and update monitoring observations, reducing manual effort and keeping review logs up to date. Teams can seamlessly review, track, and manage monitoring alongside their data. By automating routine tasks and simplifying periodic reviews, OPRA-CM focuses resources on critical risks and activities.
-
Create rules and automate record creation
-
Streamline periodic review
-
Prioritize high value activities
Reporting
H2
Unlock seamless risk communication with OPRA RAM's Advanced Reporting Capabilities. This feature is designed to streamline the way you share and communicate risk information, eradicating the complexities associated with traditional document sharing. With options for generating PDFs and bespoke reports, OPRA RAM ensures that you can tailor your risk communication to meet the specific needs of your stakeholders. These advanced reporting tools not only enhance clarity and understanding but also facilitate a more efficient exchange of critical risk-related data.
Unlock seamless risk communication with OPRA RAM's Advanced Reporting Capabilities. This feature is designed to streamline the way you share and communicate risk information, eradicating the complexities associated with traditional document sharing. With options for generating PDFs and bespoke reports, OPRA RAM ensures that you can tailor your risk communication to meet the specific needs of your stakeholders. These advanced reporting tools not only enhance clarity and understanding but also facilitate a more efficient exchange of critical risk-related data.
Unlock seamless risk communication with OPRA RAM's Advanced Reporting Capabilities. This feature is designed to streamline the way you share and communicate risk information, eradicating the complexities associated with traditional document sharing. With options for generating PDFs and bespoke reports, OPRA RAM ensures that you can tailor your risk communication to meet the specific needs of your stakeholders. These advanced reporting tools not only enhance clarity and understanding but also facilitate a more efficient exchange of critical risk-related data.

Compare
Clinical trial risk management tools
Data Visualization Tools
OPRA-CM
Real-time insights
Availability of up-to-date risk scores
Trend analysis
View evolution of risks over time
Collaboration
Centralized portal to collaborate with stakeholders
Alerts
Customizable alerts
Data visualizations
Customizable data visualizations
Version control
Eradicates duplication
Assign actions
Assign actions to stakeholders
Document corrective actions
Document key information
Audit ready
Full discoverability of mitigations after closeout
FAQs
We've got the answer
Explore our FAQs for answers and expert advice on RBQM.
TESTIMONIALS
RBQM Success Stories
Hear directly from our partners about how we've helped them.


What truly set TriTrials apart from other vendors was their unparalleled commitment to a quality deliverable helping us effectively apply a novel approach to our operations. They achieved a deep understanding of our objectives by partnering with us on a rigorous change management process which culminated in a shared vision for the integration of their risk assessment and central monitoring solution.

The Worldwide Clinical Trials team is pleased with our continued partnership with TRI and we are excited about the milestone of having 150 clinical trials in TRI's Risk-Based Quality Management platform. This is a key benefit for our customers as it improves data quality, trial efficiency, and patient safety.
RBQM NEWS
Clinical trial quality news and views
Subscribe to get the latest RBQM insights sent to your mailbox.
The Quality Issue – January 2025
Happy New Year, and welcome to our first Quality Issue of 2025! It this edition, we spotlight some of the most important developments in clinical research and Risk-Based Quality Management…
Dose Optimization in Oncology: Advancing Clinical Trial Quality through Risk-Based Approaches
The complexities of oncology drug development have long posed significant challenges, particularly in determining the optimal dose that balances therapeutic efficacy with patient safety. The FDA’s recent guidance on dose…
TRI Joins Veeva’s Product Partner Program
Partnership will drive quality and efficiency in clinical trials through enhanced risk-based quality management integration. [Cambridge, UK – 17.10.2024] – TRI, a leading Risk-Based Quality Management (RBQM) software provider for…









